INTRODUCTION
The shoulder has always been one of the most unstable and frequently injured joints within the body (1). Tendon pathologies of the shoulder are an incredibly common finding in musculoskeletal medicine; accounting for nearly 50% of all major shoulder injuries (2).
The prevalence of rotator cuff tendinopathies is thought to be as high as 26% (3) and the supraspinatus tendon is the most common site of tendinopathy in the shoulder (4).
Pain at this joint is the main symptom that initiates the patient to seek medical treatment and is reported to affect up to a third of the population at some point in their lives (5).
Given that we use the shoulder complex to control and place the entire forearm and hand, impairment to the freedom of movement at this joint has a significant impact on our ability to work and carry out daily tasks (6). Consequently, the primary goals of the medical clinician should be to eradicate this pain, address the root cause of the pathology and restore quality of life.
Historically, the literature has discussed the vascular changes leading to pathology within the chronic overuse tendinopathy (7)(8)(9)(10)(11)(12)(13). However, the demographic of sedimentary patients suffering from rotator cuff tendinopathies shows that underloading the tendon could be equally disruptive to tendon homeostasis (14).
A potential correlation between cervical spondylosis, denervation of C5 and C6 and rotator cuff tendinopathy is thought to be forming. C5 and C6 have been highlighted as an exceedingly common location of cervical spine pathology (7). Furthermore, the association of the suprascapular nerve and supraspinatus tendinosis has sparked much debate (15)(16)(17)(18)(19)(20)(21)(22)(23)(24)(25)(26). However, until recently much of the literature has focused on the interaction of denervation of the suprascapular nerve and massive rotator cuff tears post-surgery. The purpose of this article is to present an alternate theory to the mechanism of tendinopathies in the shoulder, based upon the physiological effects of denervation of the suprascapular nerve.
Rotator cuff tendon anatomy, physiology and surrounding structures:
The rotator cuff is comprised of the supraspinatus, infraspinatus, teres minor and subscapularis (6). These four muscles originate from the superior, anterior and posterior aspects of the scapula, respectively (6). These tendons blend to form a confluent aponeurotic tendon that inserts onto the tuberosities of the humeral head; collectively they provide range of motion to the glenohumeral joint, but also stability and sensory motor control (27). The supraspinatus and infraspinatus amalgamate 15mm proximal to insertion on the humerus (6). In a similar fashion, the infraspinatus and teres minor are fused just prior to the musculotendinous junction and the supraspinatus blends with the subscapularis proximally at the bicipital groove (forming a tunnel for the biceps tendon) (6). Collectively, this interwoven and blended infrastructure of tendons improves the shoulder’s resistance to degradation (28).
Tendons are primarily composed of collagen and water (55% by weight) (6). The major collagen is type I and small concentrations of proteoglycans and tenocytes are considered normal (6). As they connect muscle to bone, these structures are constantly subjected to varying load and tension (29); this is especially relevant as the rotator cuff has the largest range of motion to perform of all the tendons in the body (6). During movement the force exerted has a reciprocal effect on the tendons at either end of the muscle; essentially as the origin-side tendon is stretched into range, the insertion-side tendon is compressed, and vice versa (6). For example, during abduction the joint-side fibres of the supraspinatus are compacted, and the bursal-side fibres elongated.
The asynchronous nature of this sheering motion is thought to predispose the tendon to degeneration (6). Furthermore, fibrocartilage is found at all tendon attachments to the bone, typically 0.5-0.7mm in length (30). The fibrocartilage of the supraspinatus is the area most commonly damaged (31)- understandably described as the “critical zone” by Codman (31) because it is 20mm long in this muscle. This vast surface area is a lot of structure to be predisposed to degeneration and it makes the supraspinatus tendon the most common rotator cuff tendinosis (4)
Of all the structures at the shoulder joint, the musculotendinous rotator cuff and subacromial bursae are the most common perpetrators of pain (6). The bursae are sacks of fluid (filled with synovial cells and areolar, fibrous, and adipose tissues) with the primary function of reducing friction during movement of the joint (6). Of the bursae at the shoulder, the subacromial bursae is the largest and is innervated by the suprascapular nerve (C5, C6) (32). There is a strong positive correlation between the onset of shoulder pain and the presence of cytokines, proinflammatory and pain chemicals in the bursal tissue (6). Given the close proximity of the subacromial bursae to the rotator cuff tendons (33), it is likely pathologies of the two maybe inextricably linked.
An examination of the patient with rotator cuff tendinosis commonly finds the upper fibres of the trapezius to be extremely taut, compared to the normalised side (34). Interestingly, a great number of authors have found mechanical shoulder pain exacerbated by excessive scapular elevation to correlate with high concentrations of pain-mediating, inflammatory and matrix-modifying proteins in the bursal sacks; the resultant effect is a catabolic drive within the collagen molecules (35)(36)(37)(38)(39)(40)(41). This suggests a relationship between overactive upper trapezius fibres (observed in excessive scapular elevation) and the onset of pathological bursae. Clinical studies have highlighted the relevance of the bursae in shoulder tendinopathy; however, further study is essential to understand the histological relationship between tendinopathies of the rotator cuff, bursal pathology and shoulder pain.
What is a supraspinatus tendinopathy?
What is a tendinopathy? The term “tendinosis” was curated by Puddu et al. (42) in 1976. It is proposed that a loss of collagenous architecture, accompanied by hypercellularity, increased glycosaminoglycans and excess amorphous mucinous material, form histologic degenerative changes within the tendon (43). When examined with a light telescope, a pathological tendon will differ greatly from the norm (7). A chronic tendinopathy presents with impaired collagen continuity, increased ground substance, increased cellularity and increased vascularity (7). Unlike tendinitis, a tendinopathy is devoid of inflammation and instead the increased vascularity makes excess platelets coagulate and form calcified deposits; these chronic changes reduce functionality of the tendon considerably (43).
Neer (44) originally described three stages of rotator cuff tendon degeneration:
Stage I: observed in patients below the age of 25, classified by oedema and haemorrhage of the tendon and bursae.
Stage II: occurring in patients 25-40 years of age, identified by either partial of full thickness fibrosis or tendonitis.
Stage III: involving partial or complete tearing of the rotator cuff, seen in patients over the age of 40.
The most widely accepted cause of rotator cuff tendinopathies is repetitive overhead motions, resulting in irritation of the tendon and consequent attritional changes (45). The supraspinatus passes under the anterior edge of the acromion and adjacent to the acromioclavicular joint; when moving through overhead range of motion this space, the subacromial gap, is closed down (44). Therefore, the supraspinatus tendon (and the long head of biceps to a lesser extent) are subjected to abrasive forces with every abduction of the shoulder (44). A shoulder impingement is defined as the mechanical abrasion and compression of the rotator cuff structures found inferior to the coracoacromial arch (44). The supraspinatus tendon is the most commonly impinged (46). In an impingement pathology of these tendons, the medical professional should expect to find the impingement special tests (Neer sign (44), Hawkins Kennedy test (47), etc.) non-specific; this is due to the nature of these special tests compressing and aggravating all structures of the subacromial space. To differentiate between pathology of the long head of biceps tendon and the rotator cuff tendons, palpation is essential (7). However, it should always be considered that MRI is the gold standard for determining diagnosis of tendinopathies at the shoulder (8).
The position of the acromion is an important factor in impingement syndrome; the types of acromion are defined by shape: type 1 (flat), type 2 (curved) and type 3 (hooked) (48)(49). Enthesophyte (bony spur) formation at the coracoacromial ligament insertion on the acromion creates a type 2, progressing to type 3 (50). The ossification of these fibres is thought to be resultant of the tensile forces associated with impingement (45)(51)(52). When the humeral head translates anteriorly, as seen in hyper-kyphotic postures (53), the coracohumeral ligament is anatomically stretched as the acromion migrates away from the coracoid process; this increased tension may amalgamate in traction-type bony spurs(54). Meyer (55) first proposed how mechanical attrition of the enlarged acromion on the rotator cuff tendons may result in tendinopathy. Furthermore, Neer (45) emphasised that the supraspinatus tendon inserts anterior to the coracoacromial arch and documented that enthesophyte excrescences could compress the tendon. In all, anatomic variations in the shape of the acromion are thought to be an etiologic factor of impingement disease and rotator cuff tendinopathy.
Of 420 cadaveric shoulders analysed by Nicholson et al. (56) 68% had an abnormal morphologic acromion type 2 or 3. Despite popular belief, analysis of the data revealed no consistent statistically significant impact of age on type of acromion (56). To quantify the relationship of lateral extension of the acromion to the proportion of the individual shoulder, critical shoulder angle has been defined (CSA). CSA is simply the angle created between the inferior to superior glenoid margins and a line from the inferior glenoid to the lateral aspect of the acromion (57). Large CSA (defined by Moor et al. (57) as 38°) is associated with rotator cuff tendinopathies and tears (57)(58)(49). A large CSA is thought to destabilise the glenohumeral joint in thoracohumeral abduction, with the largest effects observed at the middle of this range (58). Gerber et al. (58) found the supraspinatus fibres were recruited up to 33% more than average to provide stability to the joint in abduction; this extra strain could put excess tension on the tendon and result in accelerated degeneration. However, it should be considered that this study included substantial anatomic simplification; no passive anatomical structures or muscles outside of the deltoid and rotator cuff were considered in this model. In all, the morphological edge of the acromion is widely associated with rotator cuff tendinopathies but the bearing of this anatomical abnormality in the pathophysiology of tendinopathies could be greater than just the attritional forces described previously (45)(55).
Seitz et al. (59) described the etiology of rotator cuff tendinopathies as multi-factorial, attributing to the various extrinsic and intrinsic mechanisms proposed. Extrinsically, the anatomical construction of the subacromial space predisposes the tendons to injury in patients with protracted scapula (44). A combination of scapula protraction, the shearing forces applied through the tendon during muscle contraction (6) and the exposed fibrocartilage “critical zone” (31), make a clear extrinsic mechanism of supraspinatus tendonitis. However, whilst acute irritation and inflammation observed in tendonitis is plausibly the manifestation of these extrinsic factors, chronic tendinopathies have the input of many other physiological changes. In order to explain the histological deviations of the chronic tendinopathy, a more complex mechanism is alluded to. Previously, tendon neovascularisation, resulting in coagulation of platelets into calcification (43), has been attributed to the intrinsic tissue healing response (60). However, there has been research to suggest that this mechanism is debatable; some authors have instead suggested there is a deficient vascular supply to the chronic tendinopathy (9)(10)(11)(12)(13). The evidence of increased vascularisation within chronic tendinopathies is overwhelmingly inconsistent. In all, there is much still to be defined within the plethora of tendinopathy theories; a more concise understanding of the origin of this pathology could have major implications to treatment in musculoskeletal medicine.
What is the relationship between the suprascapular nerve and rotator cuff tendinopathies?
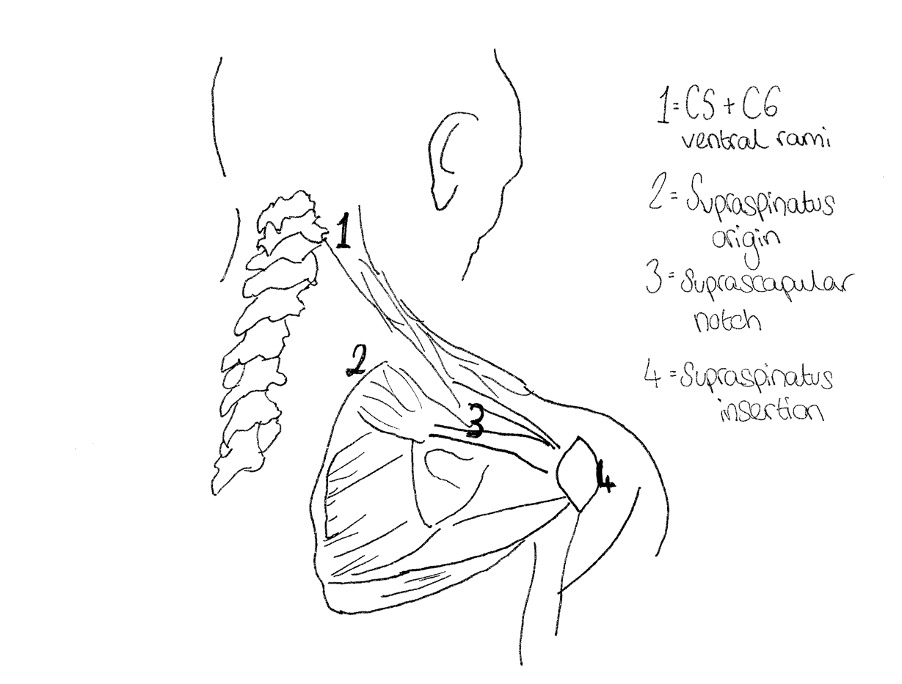
For many patients with rotator cuff pathology, superior translation of the humeral head on the glenoid fossa occurs (53), possibly as a result of the degenerative changes. However, this anatomical malfunction also frequently presents in patients with excessively kyphotic posture; this is a result of the associated protraction of the scapula and lengthening of the neck flexor muscles (61). The suprascapular nerve is located at the upper trunk of the brachial plexus at Erb’s point, branching down through the suprascapular notch of the scapula (which is bridged by the transverse scapular ligament) (1). It supplies the supraspinatus and infraspinatus by carrying the fifth and sixth cervical nerve roots (1) (see figure 1).Therefore, when this anterior translation of the humeral head occurs, the suprascapular nerve is uncharacteristically stretched. In the 2013 study, Beeler et al. (62) found that damage to the suprascapular nerve resulted in fatty infiltration and atrophy of the supraspinatus and/ or infraspinatus muscles. Many authors have started to link suprascapular neuropathy with tendinopathy of the rotator cuff (15)(16)(17)(18)(19)(20)(21)(22)(23)(24)(25)(26). Further research is now required to determine if tendinopathy degrades the nerve, or if impaired nerve signalling from the source at the central nervous system (CNS) generates the chronic degeneration seen in tendinosis.
Neuropathy of the suprascapular nerve has been shown to initiate morphological and metabolic changes in the supraspinatus muscle (20)(22)(25)(26)(63). A reduced propagation of action potentials at the neuromuscular junction, as seen in neuropathy, results in atrophy of the muscle (64). Within the fibres of the muscle, glycolysis and proteolysis increase but glucose metabolism is disrupted; this manifests into atrophy by reducing muscle fibre diameter (65). In addition to this loss of muscle elasticity and compliance, fatty infiltration (a relative increase in fat compared to protein) begins to deposit in the muscle and tendon (66). When the healthy tendon is disrupted, the respective muscle fibres shorten and pennation angle increases (67). As a result, fat is allowed to be deposited between the muscle fibres, within the interstitial spaces (67). Gumucio et al.(68) suggested that loss of muscle mass to fatty infiltration is a consequence of aging. However, it is known that tension of the suprascapular nerve can result in fatty infiltration of the supraspinatus and infraspinatus (62)(63). Given that retraction of these tendons from their bony insertion places excessive tension on the suprascapular nerve as it passes through the suprascapular notch (20), fatty degeneration of the muscle and tendon is very likely linked to neuropathy. In all, fatty degeneration has a strong correlation with chronic rotator cuff pathologies but more research needs to be done to define the pathophysiology of this (69)(70).
Albritton et al. (20) used a cadaveric study to demonstrate how retraction of the supraspinatus tendon forces an increase in tension of the motor branch of the suprascapular nerve. It is thought that when this tension manifests into neuropathy, fatty infiltration will occur and the tendon will further degenerate. Fatty degeneration is typically more severe in the supraspinatus than the other rotator cuff muscles; due to the nature of most tears starting here (71)(72). However, Kong et al. (22) found that fatty degeneration of the infraspinatus was more severe than in the supraspinatus in about 10% of patients with massive rotator cuff tears. Kong et al. (22) concluded that this result was due to suprascapular neuropathy affecting the infraspinatus in greater proportion to the supraspinatus. Kong et al. (22) also analysed the suprascapular nerve using nerve conduction studies (NCS); this showed a lower than expected prevalence of suprascapular nerve dysfunction in patients with rotator cuff pathology. However, this could be due to false negative results because of the location of the nerve at Erb’s point (1). It should also be considered that the NCS can be affected by fatty infiltration; latency is lower and hence conduction is faster (73). Therefore, the association of neuropathies with tendon degradation cannot reliably be assessed with NCS alone. Consequently, more research with larger samples of patients is required to affirm the correlation between nerve, fatty infiltration and tendon breakdown.
Suprascapular neuropathy has been described as a result of alteration to shoulder biomechanics in rotator cuff repair surgery (17). However, there is limited data on the prevalence of nerve injury before surgical intervention. The theory that suprascapular nerve injuries are present before rotator cuff repair was evidenced in the 2006 study by Mallon et al. (63). An electromyography (EMG) showed that denervation of the suprascapular nerve, a slowing of the conduction and F-potentials, was present before surgery (63). Notably, all patients had evidence of fatty degeneration (63). However, only a small sample of 8 patients were analysed and only massive rotator cuff tears included. Therefore, more research is required to determine the validity of these results and whether they could be considered representative of different severities of tendinopathy.
The mechanical factors of subacromial impingement are not the only element involved in restriction of range of motion at the shoulder joint (25). Irlenbusch and Gansen (25) compared the effect of subacromial impingement on the fast twitch, type II, and slow twitch, type I, muscle fibres of the supraspinatus and deltoid. Histologic samples showed normal distributions of both types of fibres of the deltoid but considerable reduction in the diameter of the fibres of supraspinatus (25). There were greater changes in the type II fibres than the type I and the variations were present in the acute stages of impingement (25). The results lead the authors to conclude that disturbances of the neurovascular system (such as whiplash trauma, cervical syndrome, thoracic outlet syndrome or unilateral imbalances in pressure loading) had the potential to be responsible for the onset of subacromial impingement (25). However, only the acromial aspect of the supraspinatus and deltoid muscles were analysed and therefore more research is required to clarify the effects on the other components of the rotator cuff complex. In addition to this, the idea of neurovascular involvement was only theorized, not evidenced in this study.
There is great dichotomy in the results when rotator cuff degeneration is compared to the presence of suprascapular neuropathy. Associated neuropathy of the suprascapular nerve with rotator cuff tears has been reported in between 2% to 100% of cases (74)(16)(63)(73)(75). Moreover, any direct comparison of these correlations is confounded due to the heterogeneous array of patients and injuries included. To evaluate this relationship between neuropathy and rotator cuff tendinopathy, research needs to be divided into specific samples dependent on severity of the tendinosis. Neer’s (44) 3 stages of rotator cuff disease could be used to define the groups of results.
This review proposes the theory that some patient’s rotator cuff tendinopathies are caused by reduced nerve firing from the CNS. Frequently, tendinopathies may present alongside cervical stenosis of the associated segments. The supraspinatus, the primary location of rotator cuff tendinosis (4), is supplied by C5 and C6 nerve roots (1). If the C5 and C6 segments are excessively stiff or affected by spinal pathologies as a result of poor posture (including disc bulge or other work-related upper limb pathology (7)) it could affect the nerve’s signal intensity to the muscle and tendon. C5 and C6 have been highlighted as a common location of cervical spine pathology (7). Given that the slowing of nerve conduction and F-signals in the suprascapular nerve has been found to be associated with rotator cuff tendinopathy (63), it may be theorized that this denervation causes the chronic hypervascularity seen in degrading tendons. Furthermore, when the suprascapular nerve is tight and restricted, this is thought to reduce the range of motion of the supraspinatus and infraspinatus muscles (17). When the muscle is in a restricted state, this may increase the pressure exerted on the tendon through the asynchronous nature of reciprocal tendon elongation and compression described above (6). Therefore, the restricted neural pathway creates larger shearing forces within the tendon; this may be the first step of degeneration into tendinopathy.
Tendinopathies of the shoulder are occurring more frequently than ever before in both the active and sedimentary population (76)(77). For the sedimentary population affected, no excessive overhead motions occur. Therefore, the traditional model of the “overuse” tendinopathy (repetitive overhead motions, resulting in irritation and attritional changes (45)) cannot be applied to all cases. There is a significant association between excessively kyphotic postures and the prevalence of rotator cuff tendinopathy (78). Hyper-kyphosis simultaneously creates stiffness of the cervical spine (79)and extreme strain on the rotator cuff tendons via anterior humeral head translation (53); not only does this tension the nerve pathway (53), but also compresses the nerve root (80). Moreover, hyper-kyphosis is thought to be a risk factor in increasing CSA, based upon the surge of attritional forces the associated scapulae position creates (45). Increased CSA further strains the suprascapular nerve’s anatomical pathway and closes down the subacromial space, impinging the structures (45). In conclusion, posturally induced neuropathy is a new mechanism of rotator cuff tendinopathy proposed.
The pathophysiology of rotator cuff tendinopathy is undoubtedly multi-faceted. However, considering the prevalence of both spinal pathologies and tendinopathies are increasing radically (76)(81), future research should focus on how neuropathy affects the physiological changes associated with tendinopathy. The relationship of denervation and tendon pathologies should be clarified in all tendons and their associated nerve roots. Furthermore, the mechanism of tendinopathy from kyphosis in the sedimentary patient should be more thoroughly evaluated. Given that tendons are comprised of up to 55% water (by weight) (6), future study may indicate a link between age-related cellular dehydration and tendinopathy. The implication of this research would allow clinicians to better tailor their treatment and identify the root cause of tendinopathies, as opposed to just offering symptom relief or even surgical intervention.
References:
© 2020 All rights reserved FOIMM